1~7 item / All 7 items
Displayed results
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Contact this company
Contact Us OnlineBefore making an inquiry
Download PDF1~7 item / All 7 items
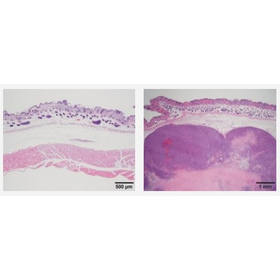
Our company conducts raw material testing, in-process control testing, and quality testing of final products for regenerative medicine products under GMP (GCPT) regulations. 【Quality Testing Items】 ■ Sterility Test (JP17 Membrane Filter Method, Direct Method) ■ Endotoxin Test (JP17 Colorimetric Method, Turbidimetric Method, Gelation Method) ■ Mycoplasma Detection Test (JP17 NAT Method using Real-time PCR) ■ Residual Testing for Antibiotics, Growth Factors, etc. (LC-MS/MS, ELISA, etc.) ■ Soft Agar Colony Formation Test, Cell Proliferation Characteristic Analysis (In Preparation) ■ Validation of Testing Methods related to the above tests In a research facility dedicated to the safety evaluation of regenerative medicine products, we conduct general toxicity tests and tumorigenicity tests using immunodeficient mice. 【Non-Clinical Testing Items】 ■ Tumorigenicity Test using Immunodeficient Mice ■ Cell Proliferation Characteristic Analysis, Soft Agar Colony Formation Test ■ General Toxicity Test ■ Safety Pharmacology Test, Efficacy Pharmacology Test ■ In Vivo Distribution Test (qPCR Method, Immunostaining) ■ Consulting for Regulatory Affairs and Applications is also available.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
In recent years, the evaluation using biomarkers has been actively incorporated at various stages of drug discovery. We possess a diverse range of analytical instruments, including the "Orbitrap Q Exactive Plus," "Triple Quad 6500+," and "MESO SECTOR S 600." [Overview] ■ Early Development: Comprehensive evaluation of various markers as pharmacological indicators ■ Application Submission: If applicable to the primary evaluation criteria, implementation starts from obtaining measurement method validation *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company conducts drug concentration analysis in biological samples (TK/PK) at all stages of pharmaceutical development under GLP compliance. In the field of biopharmaceuticals, we utilize various measurement techniques such as LC-MS/MS, ELISA, and ECL to quantitatively analyze the concentration of biopharmaceuticals in biological samples. We also possess a variety of analytical instruments that cover all aspects of the central dogma. Regarding applications, we provide services tailored to needs, ranging from omics analysis used in exploratory areas to PoM and PoC acquisition in clinical trials. 【Features of the Bioanalysis Business (TK/PK)】 ■ Provision of high-quality services ■ Comprehensive service from the establishment of measurement methods to high-throughput sample analysis ■ Facility support for all stages of pharmaceutical development (including biohazard handling and cell culture rooms) *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company conducts safety and efficacy evaluation tests for pharmaceuticals, medical devices, regenerative medicine products, cosmetics, health foods, pesticides, and chemical substances using various animals under GLP compliance, including bacteria and cells. Additionally, as an AAALAC fully accredited facility, we ensure comprehensive consideration for animal welfare. 【Non-Clinical Testing Business Content】 ■ Safety Testing Safety testing for pharmaceuticals, medical devices, food, and chemical substances. We have numerous laboratories and breeding facilities capable of accommodating small animals (mice, rats, guinea pigs) to medium and large animals (rabbits, monkeys, dogs, mini pigs). As an AAALAC fully accredited facility, all animal tests are conducted following international standards and committee review. The test results conducted at our facility can be used without issue for applications to overseas regulatory authorities and as academic science reports. - Toxicity testing and safety pharmacology testing - Special toxicity testing - Immunotoxicity testing - Genotoxicity testing ■ Pharmacological Testing - Cardiovascular - Respiratory ■ Support for various surgical procedures ■ Compliance with GMP shipping tests using experimental animals
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company uses a quality assurance process evaluated by the U.S. FDA (cGMP) to analyze and evaluate the quality of pharmaceuticals and similar products from the research and development stage to the commercial manufacturing stage using physicochemical methods, biochemical methods, and microbiological methods. We have extensive experience in responding to inspections and investigations by regulatory authorities and global quality assurance departments, with a proven track record in various GMP shipping tests and approval application tests, including physicochemical tests, microbiological tests, and biochemical tests of biopharmaceuticals. [Features] ■ Abundant experience and accumulated know-how *For more details, please download the PDF or contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company conducts drug concentration analysis in biological samples (TK/PK) at all stages of pharmaceutical development under GLP compliance. We have introduced advanced sensitivity mass spectrometry equipment (LC-MS/MS) and high-resolution mass spectrometry equipment such as the Orbitrap Q Exactive. Additionally, for the measurement of pharmaceuticals such as antibodies and ADCs, we have implemented Sector Imager Gyrolab, providing highly sensitive and selective analytical methods for various pharmaceuticals in biological samples. [Features] - Provision of high-quality services - Consistent service from method development to high-throughput sample analysis - Facility support at all stages of pharmaceutical development (biohazard compliance and cell culture rooms) *For more details, please download the PDF or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We will flexibly respond to customer needs, such as insufficient internal resources for the number of development projects and the desire to hear objective opinions that can assist with applications, and we will support various tasks related to research and development and approval applications.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration