Quality testing and non-clinical safety testing of regenerative medicine products.
Staff with highly specialized skills support research and development activities for regenerative medicine products!
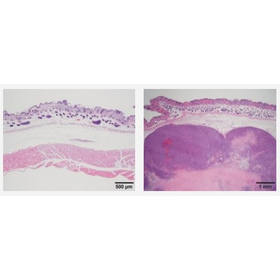
Our company conducts raw material testing, in-process control testing, and quality testing of final products for regenerative medicine products under GMP (GCPT) regulations. 【Quality Testing Items】 ■ Sterility Test (JP17 Membrane Filter Method, Direct Method) ■ Endotoxin Test (JP17 Colorimetric Method, Turbidimetric Method, Gelation Method) ■ Mycoplasma Detection Test (JP17 NAT Method using Real-time PCR) ■ Residual Testing for Antibiotics, Growth Factors, etc. (LC-MS/MS, ELISA, etc.) ■ Soft Agar Colony Formation Test, Cell Proliferation Characteristic Analysis (In Preparation) ■ Validation of Testing Methods related to the above tests In a research facility dedicated to the safety evaluation of regenerative medicine products, we conduct general toxicity tests and tumorigenicity tests using immunodeficient mice. 【Non-Clinical Testing Items】 ■ Tumorigenicity Test using Immunodeficient Mice ■ Cell Proliferation Characteristic Analysis, Soft Agar Colony Formation Test ■ General Toxicity Test ■ Safety Pharmacology Test, Efficacy Pharmacology Test ■ In Vivo Distribution Test (qPCR Method, Immunostaining) ■ Consulting for Regulatory Affairs and Applications is also available.
Inquire About This Product
basic information
Highly skilled personnel with specialized technology will support various research and development tasks. *For more details, please refer to the PDF document or feel free to contact us.*
Price range
Delivery Time
Applications/Examples of results
For more details, please feel free to contact us.
catalog(1)
Download All CatalogsCompany information

CMIC Pharma Science Co., Ltd. CMIC Bioreseach Center, Kobe Laboratory, Sapporo Laboratory
Our company, as a Non-Clinical CRO, provides solutions in the non-clinical field throughout the entire product lifecycle, from research and development stages to commercial stages, including preclinical research, bioanalysis, CMC quality analysis, analysis/measurement of biopharmaceuticals across these areas, consulting, medical writing, and more for pharmaceuticals and medical devices. In close collaboration with our group companies, we fully support our customers' value chain from product development to manufacturing, sales, and marketing across the US, Europe, and Asia. Please feel free to contact us if you have any inquiries.