1~44 item / All 44 items
Displayed results
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Contact this company
Contact Us OnlineBefore making an inquiry
Download PDF1~44 item / All 44 items

We will hold a webinar titled "Fundamentals and Applications of MicroED Crystal Structure Analysis Part 2 - Determining Absolute Stereochemistry Using MicroED." This time, referred to as "Part 2," we will introduce the determination of "absolute stereochemistry" while fully considering the effects of multiple scattering of electrons. This method is an excellent technique that demonstrates remarkable power in determining absolute stereochemistry in molecules composed solely of light elements. As a case study, this webinar will report on the determination of absolute stereochemistry using MicroED for complex natural products and synthetic pharmaceutical raw materials. [Event Overview] ■ Date and Time: October 15, 2024 (Tuesday) 14:00 - 14:45 ■ Participation: Free ■ Venue: Webinar held via Microsoft Teams *For more details, please refer to the related links or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We are pleased to announce that our company will be hosting the third installment of our webinar series titled "Case Studies of Analytical Methods in Biologics/Antibody Drugs." In light of the growing development of biopharmaceuticals, our company has been strengthening its biopharmaceutical development capabilities. During this time, we have received numerous analytical project requests from our customers. In this webinar, we will introduce analytical methods used for quality control, using antibody drugs as examples, based on the knowledge we have cultivated thus far. We sincerely look forward to your participation. 【Overview】 ■ Date and Time: February 16 (Thursday) 11:00 AM - 11:30 AM ■ Format: Online (Teams) ■ Participation: Free *For more details, please refer to the related links or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We are pleased to announce that our company will be hosting a webinar titled "Crystallization Techniques Supporting Process Chemistry and Physical Property Research." In this webinar, we will explain the nucleation phenomena underlying crystallization, which is crucial in various contexts, and discuss our approach at SpiraPharma. Additionally, we will cover the unavoidable phenomenon of polymorphism in crystallization and introduce the challenges related to its identification. We sincerely look forward to your participation. 【Overview】 ■ Date and Time: January 19 (Thursday) 11:00 AM - 11:30 AM ■ Format: Online (Teams) ■ Participation: Free *For more details, please refer to the related links or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We are pleased to announce that our company will be hosting a webinar on "Pharmaceutical Active Ingredient Manufacturing Processes Utilizing Catalytic Asymmetric Synthesis" on December 15th (Thursday). In the synthesis of active pharmaceutical ingredients for low molecular weight drugs, when the target compound is a chiral compound with an asymmetric center, utilizing asymmetric synthesis that can synthesize the desired stereoisomer with high selectivity is extremely useful in the industry. At SpiraPharma, we have received requests from various customers to examine the asymmetric synthesis of target compounds, and we are advancing the application of asymmetric reactions centered on catalytic asymmetric hydrogenation to pharmaceutical processes. In this webinar, we will introduce our efforts in asymmetric synthesis reactions. We encourage anyone interested to participate. 【Event Overview】 ■ Date and Time: December 15th (Thursday) 11:00 AM - 11:30 AM ■ Participation: Free ■ Venue: Webinar via Teams *For more details, please refer to the related links or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
A researcher from our pharmaceutical research headquarters will be speaking at the webinar hosted by the Technical Information Association titled "Mechanisms of Crystal Growth and Particle Control in Continuous Crystallization and Scale-Up," specifically in Part 3. Under the title "Considerations for Applying Continuous Crystallization and Scale-Up," we plan to explain the important points to consider when applying continuous production to solid chemical products, which has garnered attention in recent years. We sincerely look forward to your participation. 【Event Details】 ■ Date: November 7, 2022 (Monday) 10:00 AM - 5:00 PM (Scheduled speaking time: 3:00 PM - 5:00 PM) ■ Venue: Live streaming via Zoom ■ Participation Fee: 60,500 yen per person (including tax and materials) (If two or more participants from the same company register simultaneously, the fee is 55,000 yen per person) *For more details, please refer to the related links or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
At Spela Pharma, we have recently developed analytical capabilities related to the rapidly growing field of biopharmaceuticals. In "Setting Quality Testing Methods and Stability Testing for Biopharmaceuticals," we develop and establish analytical methods for the quality evaluation of biopharmaceuticals, primarily focusing on antibody drugs, conduct method validation, and perform shipping tests for clinical trial drugs. We are also capable of conducting stability tests to ensure the stability of biopharmaceuticals. In "Characterization and Equivalence Evaluation of Biopharmaceuticals," we select and evaluate appropriate analytical systems to support our clients' biopharmaceutical development. 【Main Analytical Functions (Partial)】 ■ Peptide mapping (including amino acid sequence and post-translational modification analysis by LC/MS) ■ Purity, glycan, and charge variant measurement using capillary electrophoresis ■ Charge variant measurement using ion exchange LC and isoelectric focusing capillary electrophoresis ■ Activity (potency) testing through cell assays and ELISA *For more details, please refer to the related links or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company is capable of considering injection drug prescriptions and manufacturing clinical trial drugs from the early to mid-development stages. In the summer of 2023, the manufacturing line for clinical trial drugs from early, mid, and late development to commercial production will begin operations at our group company, Iwaki Pharmaceutical Sakuragawa Factory Co., Ltd. We can provide seamless services for pharmaceutical development to commercial manufacturing, including highly pharmacologically active (highly active) drugs. We comply with GMP standards in Japan, the U.S., and Europe, as well as PIC/S GMP, and support supply to global markets, including the Japan-U.S.-Europe triad. Please feel free to contact us when needed. 【Diverse Dosage Forms】 ■ Dosage Forms: Solution formulations, freeze formulations, lyophilized formulations ■ Materials: Glass vials (3-35mL), rubber stoppers/caps (13mm, 20mm) ■ Filling: Solution filling 0.5-20mL/vial *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company realizes "formulation packaging" that meets various needs. This technology provides protection against heat, moisture, light, and impacts during transportation, as well as ensuring patient safety by preventing accidental ingestion and improving the safety of healthcare professionals. We respond to all requests related to CMC from the early stages of development to application. Please feel free to contact us when needed. [Roles (Excerpt)] ■Protection - Protection performance against heat, moisture, light, and impacts during transportation ■Compatibility - Composed of shapes and materials that do not cause physical or chemical interactions with the formulation *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Sperapharma Co., Ltd. will hold a seminar at the booth during the 24th Interphex Japan, which will be held at Tokyo Big Sight. The booth seminar will introduce our wood material development and contract manufacturing services, distinctive analysis services, and JITSUBO's unique peptide synthesis technology. If you wish to have a meeting during the event, please contact our sales representative or use the inquiry form on our website. We sincerely look forward to your visit. 【Event Overview】 ■ Event Name: 24th Interphex Week Tokyo ■ Dates: July 13 (Wednesday) to July 15 (Friday), 2022, 10:00 AM to 6:00 PM (ends at 5:00 PM on the final day) ■ Venue: Tokyo Big Sight (3-11-1 Ariake, Koto-ku, Tokyo 135-0063) ■ Booth Number: 22-68 (West Hall 2) ■ Exhibition Invitation Ticket (Free; if you do not have an invitation ticket, the admission fee is 5,000 yen per person) *For more details, please refer to the related links or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Gastric retention drug delivery systems (GRDDS) are a formulation technology that retains orally administered formulations in the stomach to gradually release the drug, thereby delaying the transition of the drug to the small intestine and sustaining drug absorption. However, drugs that are difficult to dissolve or unstable in acidic environments have been considered unsuitable for GRDDS. In this study, we designed a GRDDS combined with a self-microemulsifying drug delivery system (SMEDDS) for drugs that have issues with solubility and stability in the stomach.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
At "CPhI Japan 2022," we held a presentation in our booth regarding ICH M7 compliance from the Development and Analytical Research Headquarters. We introduced the day's content, including "About ICH M7," "For the Development of Trace Analysis Methods," and "Examples of Method Validation." Please take a moment to read it. [Contents] ■ About ICH M7 ■ For the Development of Trace Analysis Methods ■ Examples of Method Validation ■ Conclusion *For more details, please refer to the related links page or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We will be exhibiting at the 24th Interphex Japan held at Tokyo Big Sight. At this exhibition, we plan to broadly introduce the services that are available through Spira Pharma and the Astena Group. If you wish to have a meeting during the event, please contact our sales representative or use the inquiry form on our website. We sincerely look forward to your visit. 【Event Overview】 ■ Event Name: 24th Interphex Week Tokyo ■ Dates: July 13 (Wednesday) to July 15 (Friday), 2022, 10:00 AM to 6:00 PM (ends at 5:00 PM on the final day) ■ Venue: Tokyo Big Sight (3-11-1 Ariake, Koto-ku, Tokyo 135-0063) ■ Booth Number: 22-68 ■ Free Exhibition Invitation Ticket *If you do not have an invitation ticket, the admission fee is 5,000 yen per person. *For more details, please refer to the related link page or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Sperapharma will exhibit at the "2022 Summer Symposium of the Japan Society of Process Chemistry" held in Toyama Prefecture at the end of June. We will introduce our company overview and contract services such as active pharmaceutical ingredient process development and manufacturing. We sincerely look forward to your visit to our booth. 【Event Overview】 ■ Name: 2022 Summer Symposium of the Japan Society of Process Chemistry ■ Venue: Toyama Prefectural Hall (4-18 Shin-Sogawa, Toyama City, Toyama Prefecture) ■ Booth Number: 15 (3rd Floor) ■ Event Dates and Times - Symposium: June 30, 2022 (Thursday) to July 1, 2022 (Friday) 9:00 AM to 6:00 PM (tentative) - Corporate Exhibition: June 30, 2022 (Thursday) to July 1, 2022 (Friday) 10:00 AM to 5:00 PM (tentative) *For more details, please refer to the related link page or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Sperafarma exhibited at CPhI Japan 2022 (International Pharmaceutical Development Exhibition, April 20-22, Tokyo Big Sight) and welcomed many customers to our booth. As a CDMO capable of providing a wide range of services from early-stage R&D to the manufacturing of clinical trial drugs, commercial manufacturing, and raw material procurement, we will accelerate our support for pharmaceutical development. We would like to express our gratitude to everyone who provided us with opportunities for exchanging opinions and sharing information. 【Overview of Presentations at the Booth】 ■ Screening technologies required in active pharmaceutical ingredient research - catalyst selection, crystallization - ■ Mutagenic impurities in pharmaceuticals (active pharmaceutical ingredients) - development of testing methods and analysis examples - *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
One of the distinctive technologies that Supera Pharma has inherited from Takeda Pharmaceutical is the chiral technology that has been cultivated over many years in the process chemistry department. With the aim of developing manufacturing methods for chiral new drugs, we have engaged in research and development of asymmetric metal catalyst reactions centered around asymmetric hydrogenation reactions, chiral column technology for analysis and separation purposes, enzyme technology with high enantioselectivity potential, and diastereomer salt technology essential for establishing manufacturing processes. Our company will provide solutions tailored to customer needs by utilizing these technologies individually or in combination. *For detailed information about the columns, please refer to the related links. For more information, feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
In recent years, regulatory authorities have focused on impurities remaining in active pharmaceutical ingredients and formulations, requiring pharmaceutical companies to manage them strictly. At SPERAPHARMA, in managing PMIs, we identify not only the raw materials, reagents, and isolated intermediates used in the manufacturing process but also the expected reaction intermediates (non-isolated intermediates) and various types of related substances within the raw materials and intermediates, aiming to prevent the contamination of these impurities, regardless of their manifest or latent presence. So far, we have established over 300 analytical methods that guarantee PMI residues below 10 ppm, enabling us to meet the increasingly stringent requirements of regulatory authorities. As the difficulty of new drug development increases, we are refining our technologies to reliably contribute to solving challenges related to PMI management, responding to the needs of our customers who are dedicated to the creation of new pharmaceuticals. *For more details on the column, please refer to the related link. For further inquiries, feel free to contact us.*
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
The selection of starting materials (hereinafter referred to as starting materials) for applications is proposed by pharmaceutical companies in accordance with the general principles of ICH guideline Q11, but it is often the case that they receive frequent inquiries from authorities before approval. At SpiraPharma, when selecting starting materials, organic chemists and analytical chemists collaborate to propose which compounds are suitable as starting materials based on the manufacturing process. Additionally, when establishing management strategies for starting materials, we can obtain data to support responses to authorities through investigations of the behavior of key related substances. For our customers who are challenging the creation of new pharmaceuticals, we will continue to leverage our cultivated technology and experience while furthering our expertise. *For more detailed information on the column, please refer to the related link. For further inquiries, please feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
The candidates for the solid form of active pharmaceutical ingredients (APIs) are diverse, including free forms, salts, and co-crystals, each of which may have polymorphs. However, during the stage of narrowing down candidate compounds in drug development, it is necessary to select the solid form to be developed from these options. Generally, this process is referred to as solid form screening, but specific tasks are conducted according to the purpose, such as salt screening, co-crystal screening, and polymorph screening. In many cases, high-throughput (HT) screening systems are applied to these screenings. Our company continues to improve in order to provide better solutions for our customers, even for challenging issues such as poorly crystallizing compounds, compounds with undesirable properties, and compounds that do not crystallize, while also enhancing the efficiency of screening. *For more details, please refer to the related links. For further inquiries, feel free to contact us.*
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
In recent years, there have been cases where new compounds with extremely complex chemical structures or compounds with unknown physical property information are considered as candidates for new drugs, and Spira Pharma has seen an increase in requests for the manufacturing development and GMP production of such compounds. In response to the demand for prioritizing development speed, our company is taking on the challenge of process development for manufacturing methods that progress to scale-up, which I believe reflects the high expectations placed on our technical capabilities. How are we tackling the development of manufacturing methods for highly challenging compounds within a strict timeline? We spoke with the chief researcher of the Pharmaceutical Research Headquarters, incorporating examples. As experts in CMC research, our company provides comprehensive support for solving customer challenges, from early-stage R&D to clinical trial drug manufacturing, analysis, application support, and consulting. *For more details on the column, please refer to the related link. For further inquiries, please feel free to contact us.*
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company conducts the development of various dissolution testing devices and artificial gastric and intestinal fluids for formulations such as immediate-release tablets, combination tablets, orally disintegrating tablets, and controlled-release formulations, to support absorption prediction in the body and evaluate the functionality of formulations. You can trust us with dissolution testing-related technologies. 【Devices/Methods】 ■ USP Apparatus 1, 2 (Peak Vessel, Stationary Basket, etc.) ■ USP Apparatus 3 (Reciprocating Cylinder) ■ USP Apparatus 4 (Flow-Through Cell) ■ Disintegration Testing Machine (JP/USP/EP, for OD tablets) ■ Artificial Digestive Fluids (FaSSIF, FeSSIF, FaSSGF, FeSSGF, etc.) ■ Combination of two dissolution media (e.g., JP Liquid 1 → JP Liquid 2) *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company conducts rigorous stability tests under multiple temperature and humidity conditions, investigating the increase of related substances using various storage conditions (heat, humidity, light) to analyze decomposition characteristics and predict stability. We also have a variable temperature and humidity 9-chamber setup available. Please leave the decomposition characteristic investigations and stability predictions to us. *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company develops and establishes testing methods necessary for microbial management across the entire pharmaceutical development spectrum, sets up testing systems, and contributes to maintaining the quality of manufacturing facilities and pharmaceuticals. Leave the establishment of microbial testing methods in pharmaceutical manufacturing to us. 【Features】 ■ Establishment of microbial-related testing methods for active pharmaceutical ingredients and various formulations (sterility testing, endotoxin testing, microbial limit testing, etc.) ■ Problem-solving using rapid identification of environmental bacteria through MALDI/MS ■ Establishment of microbial-related testing methods necessary for managing the manufacturing environment *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
The video of the exhibitor seminar from CPhI Japan 2022 (International Pharmaceutical Development Exhibition, April 20-22, Tokyo Big Sight) that took place last month has been released. Whether you attended the event or were unable to attend for any reason, you can watch it without registration. The streaming period is until June 15, so please take this opportunity to watch it. If you have any questions, please feel free to contact us through the inquiry form. Our representative will respond to you. 【Streaming Information】 ■ Title: Screening Techniques Required in Active Pharmaceutical Ingredient Research - Catalyst Selection, Crystallization - ■ Company: Spira Pharma Co., Ltd. ■ Streaming Period: May 16 - June 15 ■ Viewing Method: Free *For more details, please refer to the related links or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We will introduce our representative formulation technology along with specific examples. The combination product of a proton pump inhibitor (a treatment for peptic ulcers) and low-dose aspirin has been developed and launched as a formulation containing 100mg of aspirin and 15mg of proton pump inhibitor per tablet. Its usefulness, including formulation characteristics, has been highly evaluated. We respond to all requests related to CMC from the early stages of development to application. Please feel free to contact us when needed. 【Features】 ■ Complete separation to avoid interactions between active ingredients ■ Maintenance of the formulation system equivalent to that of a single agent (BE) ■ Miniaturization while maintaining formulation strength *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We will introduce our representative formulation technology along with specific examples. The "composite preparation" has successfully developed all four approaches, including single-layer single-group tablets, single-layer two-group tablets, layered tablets, and core tablets / active ingredient coated tablets. We have a proven track record of launching these globally. We respond to all requests related to CMC from the early stages of development to application. Please feel free to contact us when needed. 【Four Approaches】 ■ Single-layer single-group tablets ■ Single-layer two-group tablets ■ Layered tablets ■ Core tablets / active ingredient coated tablets *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
The physical properties of active pharmaceutical ingredients often pose significant challenges in the formulation design of solid dosage forms. The "dry particle coating technology" masks the shortcomings of active pharmaceutical ingredients and adds high value in formulation design. It has various advantages over wet particle coating processes. Additionally, under solvent-free drying conditions, small particles are covered on the surfaces of larger particles by applying significant energy, such as impact and shear stress. By applying further force, the small particles fuse with the larger particles. We have successfully developed formulations using this technology and are applying it to investigational drugs targeting compounds in the development stage. 【Advantages】 ■ Cost-effective manufacturing ■ Formulation of active pharmaceutical ingredients that are unstable in water and heat ■ High-dose formulations/small tablets ■ Masking of odors/tastes *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We would like to introduce Spela Pharma's "Absorption Enhancement Technology." By using solid dispersions with polymers, we improve bioavailability. We have successfully developed formulations using this technology and are applying it to investigational drugs targeting compounds in the development stage. Our company responds to all requests related to CMC from the early stages of development to application. Please feel free to contact us when needed. 【Features】 ■ Improved bioavailability through solid dispersions using polymers ■ Successful development of formulations using this technology ■ Application to investigational drugs targeting compounds in the development stage *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We would like to introduce Spela Pharma's "Absorption Improvement Technology." By using nano-sized active pharmaceutical ingredients, we enhance bioavailability. We have successfully developed formulations using this technology and are applying it to investigational drugs targeting compounds in the development stage. On our website, we provide a correlation chart of particle size and surface area, as well as graphs showing plasma concentration in dogs for nano-particles and jet-milled active pharmaceutical ingredients, so please check the related links below. [Features] ■ Improved bioavailability through nano-sized active pharmaceutical ingredients ■ Successful development of formulations using this technology ■ Application to investigational drugs targeting compounds in the development stage *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We will introduce our representative formulation technology along with specific examples. The "oral disintegrating tablet" is designed to rapidly disintegrate and dissolve upon contact with saliva. It has received high praise from clinical settings not only for its efficacy but also for its formulation characteristics. On the Spira Pharma website, we have published a schematic diagram of the cross-section of enteric-coated granules, which you can view through the related link below. 【Case Summary】 ■ Designed to rapidly disintegrate and dissolve upon contact with saliva ■ Development and commercialization of oral disintegrating tablets containing enteric-coated granules ■ Highly praised in clinical settings for both efficacy and formulation characteristics *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration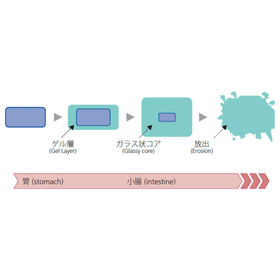
We would like to introduce our technology for "Release Control Formulations (Extended Release Matrix Tablets, Gastroretentive Formulations)." Even with reduced dosing intervals, we achieve lower Cmax and longer exposure. We have successfully developed formulations using this technology and are applying it to investigational drugs targeting compounds in the development stage. We respond to all requests related to CMC from the early stages of development to application. Please feel free to contact us when needed. 【Features】 ■ Achieves lower Cmax and longer exposure even with reduced dosing intervals ■ Successful development of formulations using this technology ■ Application to investigational drugs targeting compounds in the development stage *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company has achieved a formulation that combines two types of enteric-coated pellets, one for immediate release (IR) and the other for delayed release (DDR), to attain a longer effective plasma concentration for clinical purposes. This technology is currently applied to capsule formulations sold domestically and has received high praise from clinical settings. We respond to all requests regarding CMC from the early stages of development to application. Please feel free to contact us when needed. 【Features】 ■ Achieves longer exposure by combining two types of enteric-coated pellets ■ Releases the active ingredient faster under neutral conditions than under acidic conditions, maintaining high plasma concentration ■ Applicable to oral morphine extended-release formulations *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
The selection of the solid form of the active pharmaceutical ingredient is an essential process that cannot be avoided in the drug development process. To crystallize compounds that are difficult to crystallize, obtain solid forms with better physical properties, and comprehensively investigate polymorphism, it is necessary to examine crystallization under various conditions. At SpiraPharma, we conduct studies focused on primary nucleation using a well-plate type high-throughput device. Additionally, in cases where a mixture of polymorphs is likely to occur, we add the obtained seed crystals to each crystallization condition and use the competitive slurry conversion method to achieve a single crystal form. Furthermore, we can provide physical property evaluations, confirm thermodynamically stable crystal forms, and propose developed crystal forms according to your requests. 【Features】 ■ Conduct studies specialized in primary nucleation ■ Possible to propose developed crystal forms based on physical property evaluations and confirmation of thermodynamically stable crystal forms *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company is capable of providing drug discovery support based on insights from organic synthesis obtained during the process research of active pharmaceutical ingredients. For example, we have developed simple synthesis methods for the basic skeletons of pharmaceutical candidate compounds (e.g., heterocycles), diverse modification methods for basic skeletons (e.g., heterocycles), and reliable functional group transformation methods. We contribute to accelerating drug discovery research through the large-scale provision of key intermediates for drug discovery and the development of synthesis methods. 【Synthesis Examples】 ■ Reagents for the synthesis of quinolines (novel vinamidinium salts) ■ Synthesis methods for kinase inhibitor scaffolds (α-carbole) ■ Synthesis methods for pyrazoles ■ Synthesis methods for naphthalenes (lignan-related natural products) ■ Conversion from phenol to aniline (efficient Smiles rearrangement) *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We have equipment related to 'flow chemistry' that can meet various needs of our customers. We offer a range of equipment for small-scale synthesis of compounds that are difficult to synthesize in batch processes, all the way to kilogram-scale production, with the capability to supply samples and raw materials. We can propose green, safe, and low-cost manufacturing methods that leverage these features. To date, we have a track record of applications including nitration reactions, bromination reactions (as illustrated in the left figure), hydrogenation reduction reactions, metal exchange reactions, and boronation reactions. Additionally, we can collaborate with customers to develop flow chemistry processes. 【Application Track Record】 ■ Nitration reactions ■ Bromination reactions ■ Hydrogenation reduction reactions ■ Metal exchange reactions ■ Boronation reactions *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
The management of active pharmaceutical ingredient quality required by ICH Q11 necessitates a deep understanding of the manufacturing process through QbD experiments. In other words, (1) selecting critical quality attributes (CQAs) of the active pharmaceutical ingredient and conducting risk assessment (FMEA), (2) extracting process critical parameter profiles (PCPPs) and evaluating them through multidimensional experiments such as quality engineering and design of experiments (DoE), and (3) extracting critical process parameters (CPPs) to optimize the manufacturing method, thereby establishing a robust manufacturing process that anticipates regulatory submissions and inquiries. On our website, we provide not only plots of predicted and measured values but also contour profiles, which you can view through the related links below. *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
In recent years, regulatory authorities have focused on impurities remaining in active pharmaceutical ingredients and have demanded strict management of these by pharmaceutical companies. In particular, the management values for potential mutagenic substances (PMIs) are often in ppm units, but SpiraPharma possesses high-sensitivity analytical techniques tailored to the characteristics of PMIs, enabling the assurance of high quality in active pharmaceutical ingredients. To date, we have established over 300 analytical methods that guarantee PMI residues below 10 ppm. [Features] ■ MS: High selectivity and specificity ■ LC/FID, UV: Wide versatility ■ GC: Good peak shape *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
By utilizing chemical engineering "scale-up simulation technology" based on accumulated experience, we can proactively resolve issues that arise during scale-up and improve the robustness and productivity of manufacturing. For example, regarding the crystallization method of a raw material that is prone to oiling down and has significant variation in crystal particle size distribution, we developed a reproducible crystallization method using this technology. Additionally, for hydrated raw materials, we thoroughly examined vacuum drying conditions and developed a method to reliably obtain high-quality raw materials in a short time even at commercial scale. 【Examples of Simulation Technology (Partial)】 ■ Concentration - Simulation of solvent composition during single distillation of mixed solvents using proprietary software - Prediction of concentration time - Proposal of suitable concentration methods ■ Crystallization - Scale-up considering stirring power and changes in supersaturation over time - Development of operational methods to reproducibly obtain desired crystal forms and particle sizes - Connecting to LCM through the development of raw materials as cocrystals or suitable salts *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We propose a suitable (quality, cost, speed, etc.) active pharmaceutical ingredient manufacturing system. Understanding the various needs of our customers, we utilize not only our own manufacturing facilities but also partner CMOs based on the accumulated domestic and international CMO profiling information. We respond to all requests related to CMC from the early stages of development to application. Please feel free to contact us when needed. 【Features】 ■ Diverse customer needs ■ Establishment of reliable manufacturing technology ■ Extensive CMO portfolio *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Sperapharma builds and proposes manufacturing methods that satisfy customer needs. The manufacturing methods for active pharmaceutical ingredients vary depending on the development stage and situation of the compounds, with different needs generally arising at different stages: speed is prioritized in the early development phase, robustness and R&D cost reduction in the mid-phase, and commercial cost competitiveness and advanced process management strategy development in the late phase. Our company designs manufacturing routes based on structure-activity relationships and has succeeded in minimizing containment processes. This has enabled flexible manufacturing, shortened production times, reduced costs, alleviated workload, and decreased environmental impact. We have illustrated examples of cost reduction and cases involving highly active compounds, which you can view through the related links below. 【Features】 ■ Implementation of manufacturing route design based on structure-activity relationships ■ Successful minimization of containment processes ■ Achieved flexible manufacturing, shortened production times, reduced costs, alleviated workload, and decreased environmental impact *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
The majority of pharmaceuticals are said to be chiral compounds. Spera Pharma provides solutions to customers' "problems" related to chiral compounds by comprehensively utilizing chiral technologies (asymmetric catalytic technology, chiral column technology, diastereomer salt technology, enzyme technology, etc.). In particular, the asymmetric hydrogenation reaction technology using metal catalysts is at a world-class level and has a proven track record of application in the production of many compounds. 【Features】 ■ Responding to a wide range of needs from the development of novel asymmetric reactions to GMP manufacturing ■ Screening utilizing a library of approximately 200 types of enzymes is possible ■ Screening of diastereomer salt crystals using a library of approximately 300 types of optically active acids and bases is also conducted *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We would like to introduce our pharmaceutical formulation technology research department's business and technology. Based on the skills and experience cultivated through research and development at major domestic pharmaceutical companies, we can provide high-value-added formulation design and development tailored to the development stage (speed, cost, differentiation, novelty of formulations). We develop robust and cost-effective manufacturing processes with a focus on scaling up to high-quality, cost-competitive formulation supply and production scale, as well as preparing approval application documents (CTD Module 3). 【Features】 ■ Utilization of technologies realized and inherited by our research team at major domestic pharmaceutical companies ■ Implementation of various formulation developments with high novelty and added value ■ Extensive experience in continuous manufacturing approaches that contribute to rapid clinical trial drug production, packaging design, and packaging technology development *For more details, please refer to the PDF document or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
We would like to introduce our pharmaceutical active ingredient division (Pharmaceutical Research Headquarters) and its business and technology. We flexibly utilize a network of reliable partner contract manufacturers to build and propose an optimal active ingredient manufacturing supply chain tailored to specific purposes. With world-class pharmaceutical development technology and assured quality, we provide "the development of active ingredient manufacturing methods from 'zero' that meet global application requirements" and "a seamless and consistent active ingredient manufacturing process and quality design from preclinical stages to obtaining marketing approval." 【Features】 ■ Development of active ingredient manufacturing methods from 'zero' based on technology and know-how cultivated over many years at major domestic pharmaceutical companies. ■ Establishment of active ingredient manufacturing methods and quality design according to development stages and applications. ■ Flexible response to active ingredient manufacturing requests. ■ Accurate support for application processes (clinical trials and commercial use). *For more details, please refer to the PDF materials or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
【Exhibition Information】 We will be exhibiting at the 23rd Interphex Japan! Sperapharma will be exhibiting at the 23rd Interphex Japan (December 8-10, 2021, Makuhari Messe). Since becoming Astena HD in June of this year, we have significantly expanded the contract services we can offer, and we would like to introduce them to you. We are also planning seminars within our booth, so please be sure to check those out as well! For seminar information, please refer to the related links. All of our staff sincerely look forward to welcoming you.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration
Our company supports the creation of excellent pharmaceuticals and groundbreaking drugs with the expertise of CMC researchers who possess outstanding skills and passion. We handle all CMC processes from research and development to application, providing services tailored to our customers' needs. As the difficulty of new drug development increases, we refine our cultivated technologies further, bringing new developments to pharmaceutical research and development, and contributing to the realization of a healthier society through the creation of excellent new drugs. 【Features of the Active Pharmaceutical Ingredients Division】 ■ World-class asymmetric catalyst technology ■ Manufacturing method establishment according to development stages ■ Scale-up simulation technology ■ High-sensitivity analytical techniques supporting impurity management ■ Establishment of robust manufacturing methods utilizing QbD experiments, etc. *For more details, please download the PDF or feel free to contact us.
Added to bookmarks
Bookmarks listBookmark has been removed
Bookmarks listYou can't add any more bookmarks
By registering as a member, you can increase the number of bookmarks you can save and organize them with labels.
Free membership registration