Oral absorption analysis software 【Pharmaceutical simulation video available!】
gPROMS FormulatedProducts Oral Absorption Analysis Tool
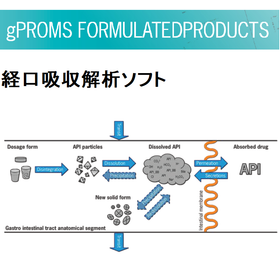
A decisive factor in determining the formulation or injection solution! It is possible to simulate oral absorption during active ingredient development.
As part of the gPROMS Formulated Products library, there is an oral absorption analysis tool. In the analysis of oral absorption of pharmaceuticals, it is possible to quantitatively understand the effects of drug administration schedules, feeding conditions, and formulation on oral absorption. 【Features】 ■ Steady-state and dynamic (transient) simulation ■ Handling of multiple recycle streams ■ Optimization of plant design and operating methods ■ Identification and estimation of process equipment performance from test data ■ Graphical representation of batch plant operating procedures ■ Integration with crystallization process analysis library ■ Integration with oral absorption analysis library *For more details about the product, please visit our website.
Inquire About This Product
basic information
Modeling and simulation of solid handling processes are expected to yield the following effects: 1. Advanced quality monitoring and management (PAT, QbD, RTRT) 2. Reduction of development and manufacturing time, and space-saving of equipment 3. Adaptation to small-batch, diverse production 4. Ease of scale-up and scale-down 5. Improvement of product quality (particle size distribution) 6. Increased production volume in existing plants 7. Support for the formulation of flexible operating guidelines in response to production demands 8. Identification of individual process and solid handling equipment characteristics We provide support from demo tests to utilization follow-up.
Price range
Delivery Time
P3
※This is the delivery date after the licensing agreement. If you have any other questions, please feel free to contact us.
Applications/Examples of results
<Features of Continuous Production Process> (1) Reduction of human errors and avoidance of stock-out risks through early detection of quality defects (2) Reduction of manufacturing costs and ease of changing or relocating manufacturing sites (3) Application to generic pharmaceuticals and personalized medicine (4) Reduction of costs related to manufacturing and storage, etc.
catalog(10)
Download All Catalogs

News about this product(1)
Company information
Founded in 1997 by the gPROMS development team at Imperial College London, PSE became part of the Siemens Group in 2019 and has been providing products and services in Japan as Siemens Corporation since 2023. gPROMS features a powerful equation-based computation engine that differs from traditional simulators, allowing for seamless handling of both steady-state and dynamic simulations. This enables the effective scaling up of models adjusted with experimental data from batch processing for the design and operational optimization of continuous processing plants. With gPROMS's excellent capabilities in custom modeling, parameter estimation, and optimization, it is possible to quickly build optimal manufacturing processes, resolve issues, and achieve optimization.














![[Online Event] gPROMS Software Product Training](https://image.mono.ipros.com/public/product/image/c84/2000406086/IPROS18384369678019508803.png?w=280&h=280)




![[ISCEF Use Case] Tunnel and Excavation](https://image.mono.ipros.com/public/product/image/9f7/2000337139/IPROS8311947278188742537.png?w=280&h=280)




